Abstract
Background:
Granulocyte colony stimulating factor (G-CSF) to prevent neutropenic fever is a well-accepted standard of care to minimize the risk of neutropenic fever during consolidation chemotherapy for acute myeloid leukemia (AML). Although prospective, randomized clinical trials of filgrastim versus depot pegfilgrastim demonstrated biologic equivalency, retrospective studies in patients with solid tumors suggest the use of pegfilgrastim results in reduced rates of admission for neutropenic fever. Patients undergoing AML consolidation may be at higher risk for admission, as with solid tumors. Biosimilars for filgrastim (filgrastim-sndz and tbo-filgrastim) and pegfilgrastim (pegfilgrastim-jmdb) are creating market forces that may make daily administered agents an attractive alternative to pegfilgrastim, and therefore additional observational studies to clarify of the safety of daily administered G-CSF vs depot injections. We hypothesize that the use filgrastim over pegfilgrastim may result in higher hospital admission rates in AML patients undergoing consolidation chemotherapy outside the setting of prospective trials.
Methods:
Patient charts from the year 2004 through early 2017 diagnosed with AML at the Seidman Cancer Center of University Hospitals Cleveland Medical Center (UHCMC) were reviewed as a retrospective cohort study. Patient were included who received at least one cycle of high dose cytarabine during 1st complete remission, and received filgrastim or pegfilgrastim were considered available for analysis. Individual cycles of chemotherapy were assessed for potential adverse outcomes with the use of different forms of GCSF. The primary outcome of interest is hospitalization during consolidation chemotherapy. The incidence of hospitalization was analyzed using longitudinal logistic regression with generalized estimating equations for statistical inference assuming exchangeable variance-covariance structure of multiple hospitalizations from the same patients.
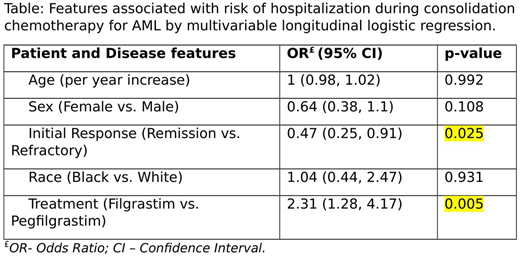
Results
436 patient charts were reviewed identifying 165 patients receiving at least one cycle of HiDAC chemotherapy. Of these, 156 patients received either filgrastim or pegfilgrastim, representing 256 cycles of high dose cytarabine chemotherapy supported with GCSF. Patients receiving filgrastims vs pegfilgrastim differed in the number of HiDAC cycles received (1.64 vs 1.97, p = 0.046), the distribution of favorable risk AML (11.9% vs 32.7%, p = 0.035). Groups did not differ based on age, sex, race, initial response to therapy, and proportion of secondary AML. After controlling the effects of age, sex, race and initial clinical response, treatment (filgrastim vs. pegfilgrastim) was significantly associated with hospitalization during consolidation therapy (p = 0.005). Specifically, the odds of having hospitalization for patients treated with Filgrastim is estimated to be 2.31 times the odds of having hospitalization for patients treated Pegfilgrastim with 95% CI (1.28, 4.17). Also, the estimated probability of having hospitalization for patients treated with filgrastim was 0.59 (95% CI: 0.43- 0.73) vs. 0.38 (95% CI: 0.27 - 0.51) for patients treated with pegfilgrastim.
Conclusions:
The use of filgrastim was found to be statistically significantly associated with and increased risk of hospitalization.
Caimi:Kite Pharma: Other: Advisory Board Participation; Celgene: Speakers Bureau; Genentech: Other: Advisory Board PArticipation, Research Funding; Kite Pharma: Other: Advisory Board Participation. Little:PCORI: Research Funding; Hemex: Patents & Royalties: Patent, no honoraria; Doris Duke Charitable Foundations: Research Funding; NHLBI: Research Funding. Malek:Sanofi: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.